Desulfovibrio desulfuricans, found in the digestive tract of both man and animals, is the most common bacteria which produce H2S under anaerobic conditions. These obligate anaerobes use sulfate as their oxygen source, ammonia as their sole source of nitrogen, and various forms of organic matter as a food supply including amino acids, carbohydrates, organic acids, etc., when in an oxygen limited environment. These reactions often take place in the slime layer on collection pipes and in the sludge of lagoons, etc.
These bacteria cannot compete well with the facultative anaerobic strains in Alken Clear-Flo® formulas, which use nitrate as a hydrogen acceptor and reproduce more quickly than the sulfur-reducing pure anaerobes.
The serious odor and corrosion problems associated with the collection, handling and treatment of domestic wastewater are primarily the result of sulfate reduction to hydrogen sulfide under anaerobic conditions, as shown by the following reactions :
|
1) 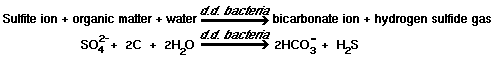
2) 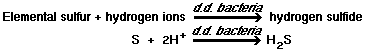
3) 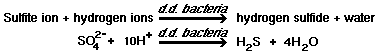 |
In the biochemical oxidation of organic matter, bacteria remove hydrogen atoms from the organic molecule and, in the process, gain energy. Through a series of biochemical reactions, the hydrogen atoms are transferred to a hydrogen acceptor. The hydrogen acceptor may be an inorganic or organic substance. Under aerobic conditions, free oxygen is the final acceptor for hydrogen, the oxygen being reduced to water. In the absence of free oxygen, combined oxygen may be used as a final acceptor of hydrogen.
1) 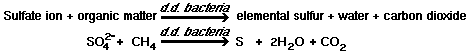
2) 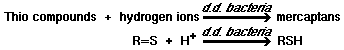
1) 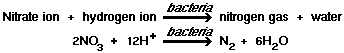
2) 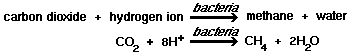
|
 |
