Thiobacillus
denitrificans and Paracoccus pantotrophus (formerly Paracoccus
denitrificans and Thiomicrospira pantotrophus) can function aerobically
or anaerobically using nitrate as a source of oxygen. Our recently reformulated
powdered product, Alken Enz-Odor
6 contains both of these species along with the obligately aerobic
sulfide and mercaptan oxidizer, Starkeya novella (formerly Thiobacillus
novellus) which does not produce acid
during oxidation and is facultatively able to degrade general BOD and a
wide assortment of fatty acids. Alken-Murray sells Starkeya novella
alone, under the name Alken Enz-Odor
14, and also includes it in a variety of other products. Most recently of all, Alken-Murray's Valerie Anne Edwards isolated a new species of heterotrophic, facultatively anaerobic, sulfide oxidizing Bacillus. Dr. Frederick Cohan of Wesleyan University has done the DNA probe work on this new species and we will be proposing the name Bacillus sulfidophilus for it. For now, the strains are listed with the name of their closest relative, Bacillus mojavensis. Three strains of this new species are currently included, singly or together, in the following Alken-Murray formulas: Alken Clear-Flo 1003, Alken Clear-Flo 1005, Alken Clear-Flo 1008, Alken Clear-Flo 1400-50x, Alken Clear-Flo 7114, Alken Clear-Flo 7139, Alken Enz-Odor 2, Alken Enz-Odor 4, Alken Enz-Odor 5, Alken Enz-Odor 6, Alken Enz-Odor 8, Alken Enz-Odor 9, Alken Enz-Odor 10, Alken Enz-Odor 12. Alken Nu-Bind, Alken Nu-Bind 2, Alken Nu-Bind 3, and Alken Treat-A-Loo 2. |
|
The following bacteria are autotrophic aerobes, requiring a sulfur source (hydrogen sulfide, elemental sulfur or thiosulfate) for energy:
| |
The following bacteria are autotrophic anaerobes, requiring a sulfur source (hydrogen sulfide, elemental sulfur or thiosulfate) for energy:
| |
With the addition of 10 to 100 ppm of sodium
nitrate (or a specialty nitrated blend like Alken 896, Alken Enz-Odor 9 or Alken Enz-Odor 10) to the prescribed dosages of Alken
Enz-Odor 8 or Alken Clear-Flo
7020 (see dosage chart, linked from product bulletin for correct
dosage), the following reactions will occur. Bacteria prefer hydrogen receptors
in the following order: oxygen, nitrate ion, and sulfate. | |
| Theoretically, in the absence of oxygen, no sulfide will be generated until all of the nitrate has been reduced to nitrogen gas. | |
1) 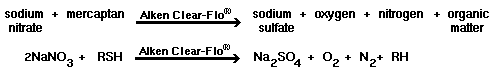 |
2) 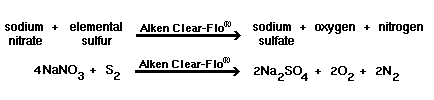 |
3) 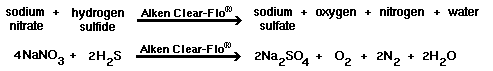 |
If pH is below 5, Thiobacillus concretivorus will perform the following reactions, in the presence of up to 7 percent sulfuric acid concentrations. They draw a carbon source from carbon dioxide. A pH above 10 will kill this strain. |
| 1) Elemental sulfur, hydrogen sulfide, thiosulfate and polythionates can be degraded by T. concretivorus to sulfuric acid, which lowers the pH to around 2. |
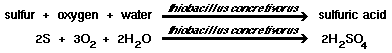 |
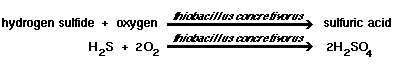 |
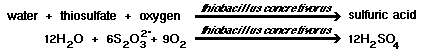 |
| 2) In a pH range of 5 to 7, Thiobacillus thioparis will establish itself and degrade hydrogen sulfide to thiosulfuric and polythionic acids. |
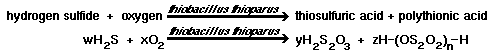 |
Controlling H2S by Combining Bonding and Degradation Another safe option for eliminating the odor from hydrogen sulfide is the application of high molecular-weight humified peat moss combined with appropriate bacteria to bind the hydrogen sulfide and degrade the source of odor. Be sure that the product you select is a humified peat moss, not leonardite and that it is not a low molecular weight product, as is commonly marketed to agriculture to enhance plant growth. A high molecular weight humified peat moss , such as that found in Alken Nu-Bind, binds hydrogen sulfide and ammonia, while increasing bacterial cell wall permeability , catalyzing higher performance and increasing activity levels from the bacteria in the product. Alken Nu-Bind is popular for solving municipal waste treatment, industrial applications, and animal farming odors.
Other alternatives for binding hydrogen sulfide and mercaptans are certain natural polymers, which also work co-operatively with bacteria. Alken Nu-Bind 2 is an example of such a product, binding mercaptans that the original product cannot, while its hydrogen sulfide binding is second only to the original Nu-Bind. For garbage cans, dumpsters, etc, there is no better product. The client's budget, regulatory demands, and composition of the waste, temperature, pH etc. will dictate the appropriate solution, whether it is bacteria alone, bacteria with nitrate, or bacteria with a binding agent.
 |
