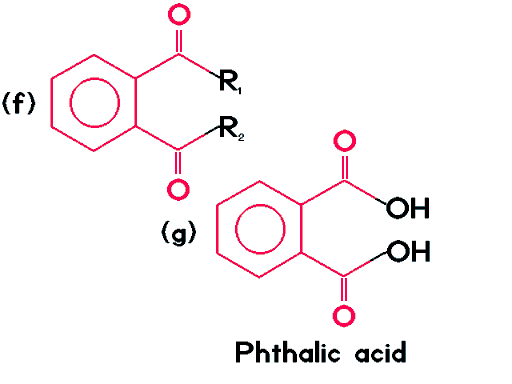
| All phthalates follow the pattern shown by (f), where R1 and R2 may represent various atoms or functional groups. The most common and simplest phthalate is phthalic acid (g). I find phthalic acid is of special interest. If one of the OH groups is neutralized with potassium hydroxide, to give an OK, instead of OH, the resulting compound is called potassium hydrogen phthalate. This compound is used as a pH 4.00 reference, to calibrate pH meter electrodes. It has exceptional buffering abilities and changes its pH very little with significant temperature variation. |
 |
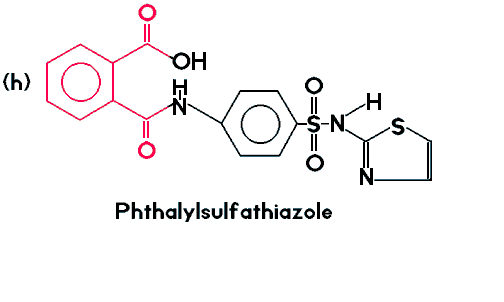
| (h) is still considered a member of the phthalate family because of the portion of the molecule shown in red. |
 |
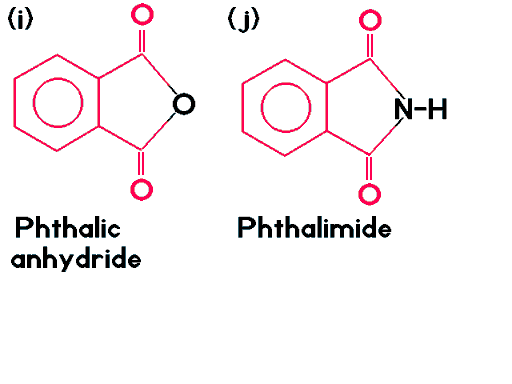
| Phthalic anhydride (i) and phthalimide (j) also belong to the phthalate family in spite of the fact that both carbonyl groups share an atom between them. |
 |
Those of our bacteria which can degrade phthalates should be able to degrade at least the phthalate portion of the molecules shown in g, h, i and j. See the University of Minnesota Biocatalysis-Biodegradation pathway for Phthalates, to see the mechanism for biodegrading Phthalates. |
